Онош, 2011, 52(052)
Long term follow-up of a group of chronic hepatitis C patients treated with anti-inflammatory drugs following initial interferon therapy
( Лекц )
Abstract
Background: A relationship between hepatocellular carcinoma (HCC) recurrence and serum alanine aminotransfer¬ase (ALT) in a group of hepatectomized patients has been reported. Another study suggested the development of HCC is more rapid in a high ALT group of hepatitis C virus (HCV)-associated cirrhotic patients. To find a relationship between ALT and HCC occurrence, we observed changes in ALT over a period of 6 years, in a group of non-cirrhotic, chronic hepatitis C (CHC) patients treated with anti-inflammatory drugs post interferon (IFN) therapy. Method: Eighty three CHC patients, with fibrosis stage 1, 2, 3 (Fl, F2, F3) who had a partial (PR) or non-response (NR) to initial IFN therapy, were treated with anti-inflammatory drugs for 6 years. Over a period of 6 years HCC developed in nine patients. Of them, one belonged to F2 and eight to F3. Within the first 2 years HCC developed among two patients in F3. Multivariate analysis revealed that in F3, the 6 year average ALT activity (odds ratio 5.59; P < 0.05) was the only significant variable associated with HCC occurrence. All other variables remained insignificant. Among the six F3 patients in whom HCC developed, the likelihood of HCC occurrence was found to be significantly higher (odds ratio 1.89; P < 0.001) in patients who showed elevated ALT activity ( > 80 IU) two or more times during the 6 year period, compared to those with ALT ( > 80 IU) for less than 2 years. Conclusion: These findings suggest that continuous elevation of ALT seems to be important for HCC diagnosis. Patients with ALT ^ 80 IU for 2 years or more are at a greater risk of HCC development. It is necessary to continue treatment with anti-inflammatory drugs, following initial IFN therapy to suppress ALT below 80 IU, to prevent HCC occurrence or delay the time of HCC occurrence in order to prolong life.
© 2002 Elsevier Science B.V. All rights reserved.
Keywords: CHC; HCV-associated cirrhosis; ALT; HCC; IFN; Anti-inflammatory drugs hepatitis C virus (HCV) develop chronic hepatitis [1] which leads to cirrhosis and subsequently hepatocellular carcinoma (HCC). Anti-viral drugs particularly IFN-a [2] seems to be the only drug that has been shown to be effective in the treatment of chronic hepatitis C, but only half of the patients respond transiently or permanently. Among the liver enzymes, ALT has been found to be a well-known marker of liver cell necrosis, since it represents the inflammatory necrosis of hepato-cytes. With animal experiments it has been shown that persistent inflammation may be a cause leading to carcinogenesis. The development of HCC in HCV associated cirrhotic patients was found to be more rapid in the persistently, high ALT group (^ 80 IU) than the persistently low (< 80 Ш) ALT group [3]. Longer treatment regimens, early response, younger age, shorter duration of disease, absence of cirrhosis, low pretreatment viral loads and infection with non-lb genotype, have all been associated with higher response in some patients [4-10]. However, longer treatment regimens with IFN are costly and have a limited period of administration. In such cases, the use of anti-inflammatory drugs such as Stronger Neo-Minophagen C (SNMC) and Ursodeoxy¬cholic acid (UDCA) and Shosaiko-to, after initial IFN therapy to lower ALT activity, may help to prevent HCC or prolong HCC development and be also beneficial for the patients in terms of cost. The efficacy of long term administration of SNMC in CHC patients to prevent HCC have been reported [11]. Other studies have shown that UDCA is capable of improving liver function, significantly ALT and AST in CHC patients [12-14]. The anti-tumor effects of Shosaiko-to in preventing HCC development in cirrhosis patients negative for Hepatitis B s antigen is also known [15]. In our present study, we observed a group of patients who had little or no response to IFN, over a period of 6 years, and who had been treated with SNMC, UDCA and Shosaiko-to to prevent or prolong the steps leading to carcinogenesis and subsequently prolong life. Our study aimed at monitoring biochemical changes during follow-up, particularly ALT and platelet changes and their relation to the subsequent development of HCC based on the staging of liver fibrosis.
2. Patients and methods
A total of 83 patients who underwent IFN treatment at Kawasaki Hospital Okayama and Okayama Medical School Hospital, Okayama, Japan, over an average period of 69 + 26 months, were studied. Patients who had HCV RNA positive in sera, who were histologically diagnosed as chronic hepatitis and had abnormal ALT ( ^ 80 IU/1) for 6 months or longer, at least 3 months prior to initial IFN therapy were included in this study.
2.1. Evaluation of clinical effect
Patients who maintained normal ALT activity (less than 40 IU) for more than 6 months and were negative for HCV RNA after the cessation of IFN therapy were taken to be sustained responders (SR), those whose ALT activity was between 40 and 80 IU and were HCV RNA positive in sera 6 months post IFN therapy, were taken to be partial responders (PR) and non responders (NR) were those who had persistent HCV viremia and ALT activity (^ 80 IU) longer than 6 months after completion of the IFN treatment. PR and NR patients were only included in this study. SR were excluded from this study as the purpose of this study was to concentrate on finding factors to improve the condition of patients who had partial or non response to initial IFN therapy. Patients with preexisting HCC at the start of this study were excluded. Those patients who died during the course of this study were also omitted.
2.2. Six-year follow-up study
During the follow up period, patients were examined by ultrasonography (US) every 6 months and if liver cirrhosis was detected, every 3 months on an outpatient basis. Computed tomography (CT) and magnetic resonance ima¬ging (MRI) were performed once a year routinely and whenever necessary. Serum ALT activity, tests for liver function, and other biochemical markers were done every month. If HCC was suspected, patients were admitted. Upon admission, further imaging techniques, angiography and liver biopsy was carried out to diagnose HCC. A total of 83 patients were followed up over a minimum period of 6 years.
2.3. Laboratory data analysis
The average of 6 years of serum ALT, PLT and other markers, starting from the beginning of this study, was calculated in all patient. Annual average serum ALT activity < 80 IU were consid¬ered low, while annual average ALT activity 2: 80 IU were considered high. The decrease in platelet count during the follow-up period was calculated from the average platelet count at the beginning of the study to the end of the study.
2.4. Therapeutic procedures
Post IFN therapy, patients were treated with the following anti-inflammatory drugs: SNMC was administered with a starting dose of 2-3 ampules (20 ml/ampule), three times a week with a max¬imum dosage of 4-5 ampules, four to six times a week. UDCA was given at a starting dose of 300-600 mg/day. with a maximum dose of 900 mg/day. Most patients received 600 mg/day. Shosaiko-to was administered at a dose of 7.0 g/day. Since the drugs were administered for the purpose of low¬ering and stabilizing serum ALT activity, their dosage was altered accordingly, except for Sho¬saiko-to.
2.5. Statistical analyses
Logistic regression analysis was done using the statistical package SAS, version 6. Results were expressed in means+ S.D. Informed consent was obtained from each patient regarding this study.
3. Results
Out of the total 83 patients, HCC developed in nine, over an average observation period of 69 + 26 months. Since HCC developed in only one out of 41 patients in Fl and F2, emphasis was placed on the F3 group. The demographic features of all the 42 patients in F3 based on the presence or absence of HCC is shown in Table 1. On univariate analysis, two variables, the average 6 year ALT activity (P = 0.021) and the decrease in platelet count (P = 0.044), were found to be significantly related to the incidence of HCC. No other variable showed any significant difference. Multivariate analysis of the significant variables in F3 was done taking HCC as the dependant variable to find the best predictor of HCC occurrence (Table 2). Results showed that the odds in favor of ALT was 5.59 (95% confidence interval: 1.29-42.30; P = 0.044). The decrease in platelet count after 6
Table 1
Comparison of the clinical profile of patients in the non-cirrhotic group with stage 3 fibrosis based on the likelihood of HCC occurrence
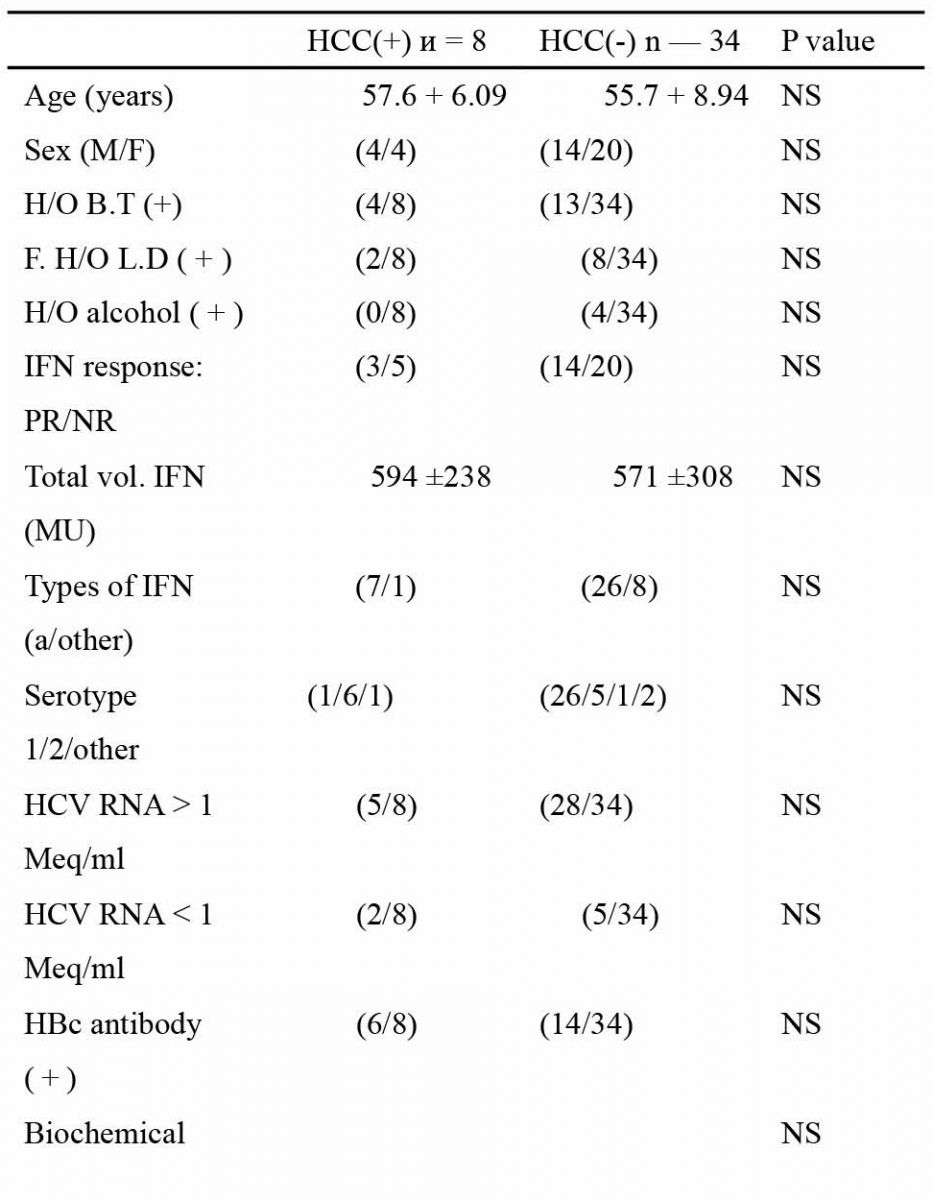
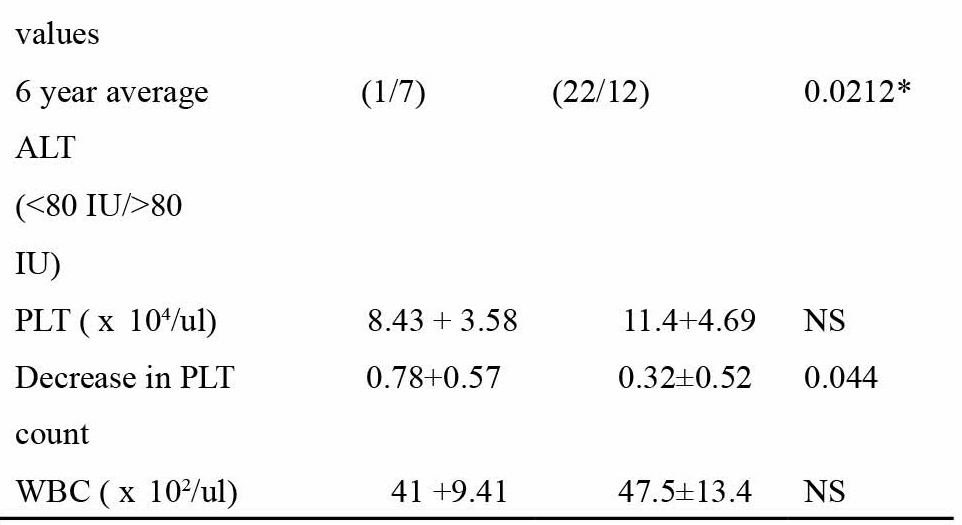
M/F, male/female; H/O, history of; LD, liver disease; IFN, interferon; ALT, alanine aminotransferase; PLT, platelet; HCC, hepatocellular carcinoma.
* Significant on univariate anlysis. Data are mean±S.D.
Table 2
Multivariate analysis showing the best predictor of HCC occurrence in non-cirrhotic CHC patients with stage 3 fibrosis
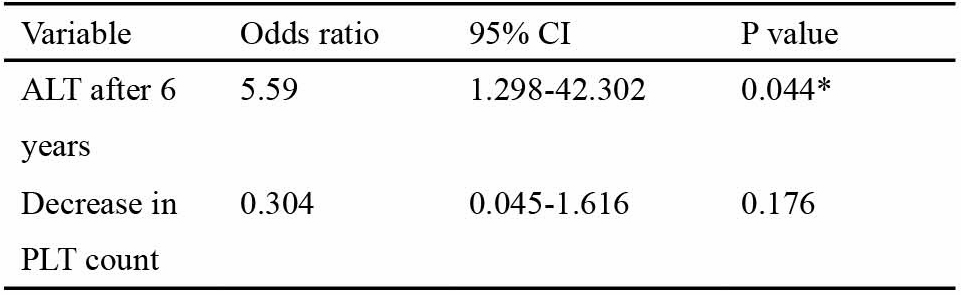
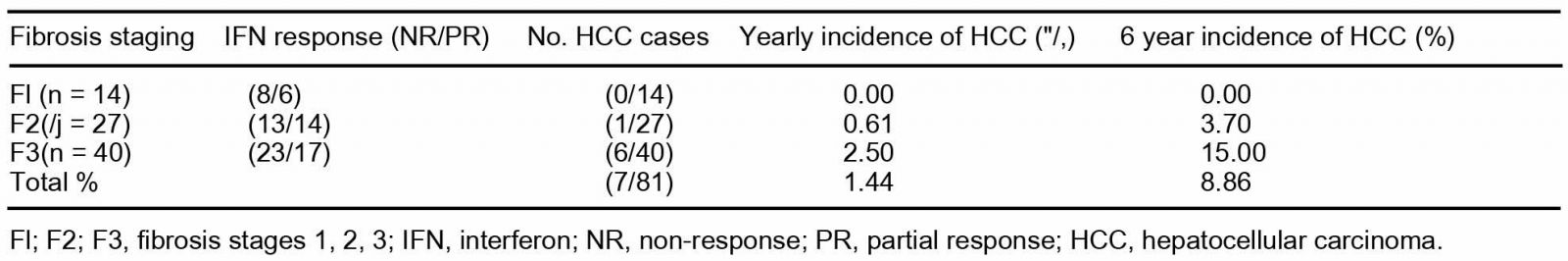
CI. confidence interval: ALT, alanine aminotransferase; PLT, platelet. years was not significant (P = 0.176). In total eight out of 42 patients developed HCC, but two patients developed HCC within 2 years, so they were not included in the following tables. Almost all patients in F3 group received combinations or single therapy of SNMC (95%), while UDCA (80%) and Shosaiko-to (30%) (data not shown). Fig. 1 summarizes the change in ALT among the 40 F3 patients during the observation period of 69 ±26 months. In total six patients developed HCC over an observation period of 6 years. Among the 23 patients whose average ALT remained below 80 IU or was ^ 80 IU only once during the observation period, one developed HCC in 6 years. One HCC patient had ALT > 80 IU twice during the follow-up period. The remaining four HCC patients showed elevated ALT > 80 IU 3 or more times during the total 6 year period. Table 3 shows the yearly and average 6 yearly incidence of HCC in F3 patients based on the different fibrosis staging. The yearly and 6 yearly incidence of HCC in Fl was 0%; in F2 was 0.61 and 3.70%; in F3 was 2.50 and 15%, respectively. The yearly incidence of HCC in F3 was four times higher than in F2. 4. Discussion
It is a known fact that many patients with chronic hepatitis C will eventually show progres¬sion of their disease after a long and symptom less course [16]. The final outcome of chronic hepatitis C is HCC with or without cirrhosis. Though about 6-8% of all HCV associated cirrhotic patients in Japan are at a high risk of HCC development [3], the non-cirrhotic group cannot be overlooked. Many studies have already shown that in HCV-associated cirrhotic patients, persistently high serum ALT is a major risk factor in the develop¬ment of HCC [3]. The rate of response to IFN has been reported to be lower in cirrhotic patients than non-cirrhotic patients, due to extensive cell da¬mage caused by cirrhosis [8,17], Normalization of ALT by IFN (even without HCV eradication) has led to the less likelihood of HCC occurrence [18]. ALT causes alleviation of hepatic necrosis and liver fibrosis, which in turn reduces the incidence of genetic abnormalities accumulating during cell division, thus lowering the risk of HCC [19-22]. However, due to side effects and cost related factors, continued IFN therapy is not feasible. In such patients the use of anti-inflammatory drugs to control ALT seems justified in lowering or stabilizing ALT, and thus delaying the conse¬quences leading to HCC. This study was aimed at observing the change in ALT activity over a certain period, in non-cirrhotic CHC patients, particularly F3, who were either NR or PR to initial IFN therapy, and who were treated with anti-inflammatory drugs to lower or stabilize ALT. In addition, this study tried to isolate predictors of HCC development in the non-cir¬rhotic group, particularly F3. Some studies sug¬gested age, sex and non-response to IFN therapy
Table 3
Incidence of HCC in CHC patients according to staging of fibrosis during
the 6-year follow-up period
were risk factors related to HCC [18]. Others have pointed out that the HCV core protein has a chief role in the development of HCC [25], that chronic hepatitis is not always a prerequisite for the development of HCC and that HCV itself has direct involvement. Nishikawa et al. have found a consistency between the genetic instability that results in high rate of mitochondrial DNA muta¬tion in cancerous liver tissue and multicentric hepatocarcinogenesis [26]. Close association be¬tween high serum ALT and more rapid recurrence of HCC in hepatectomoized patients with HCV-associated liver cirrhosis and HCC have been demonstrated by Tarao et al. [27]. In this study, particularly in F3, ALT seemed to be the only predictor of HCC occurrence.
In patients with fibrosis stage 3, the yearly rate of incidence of HCC was found to be four times more than F2, which means that F3 patients with a yearly average ALT greater than 80 IU are more likely to develop HCC compared to stages Fl or F2.
Of the nine patients who developed HCC during the course of this study, eight belonged to F3. The mechanism of HCC from CHC is still not fully known, but it is believed that the core protein of HCV might be directly carcinogenic, at least in vitro [23,24]. The possibility of pre-existing HCC can be accounted for HCC development, within the first 2 years of the observation period in the two F3 patients. Only one F3 with HCC, had an average yearly ALT < 80 IU all throughout the 6 year follow-up period. Perhaps in such a case, the presence of elevated ALT much before the study period which were not recorded, should be taken into consideration. In our study group, patients with 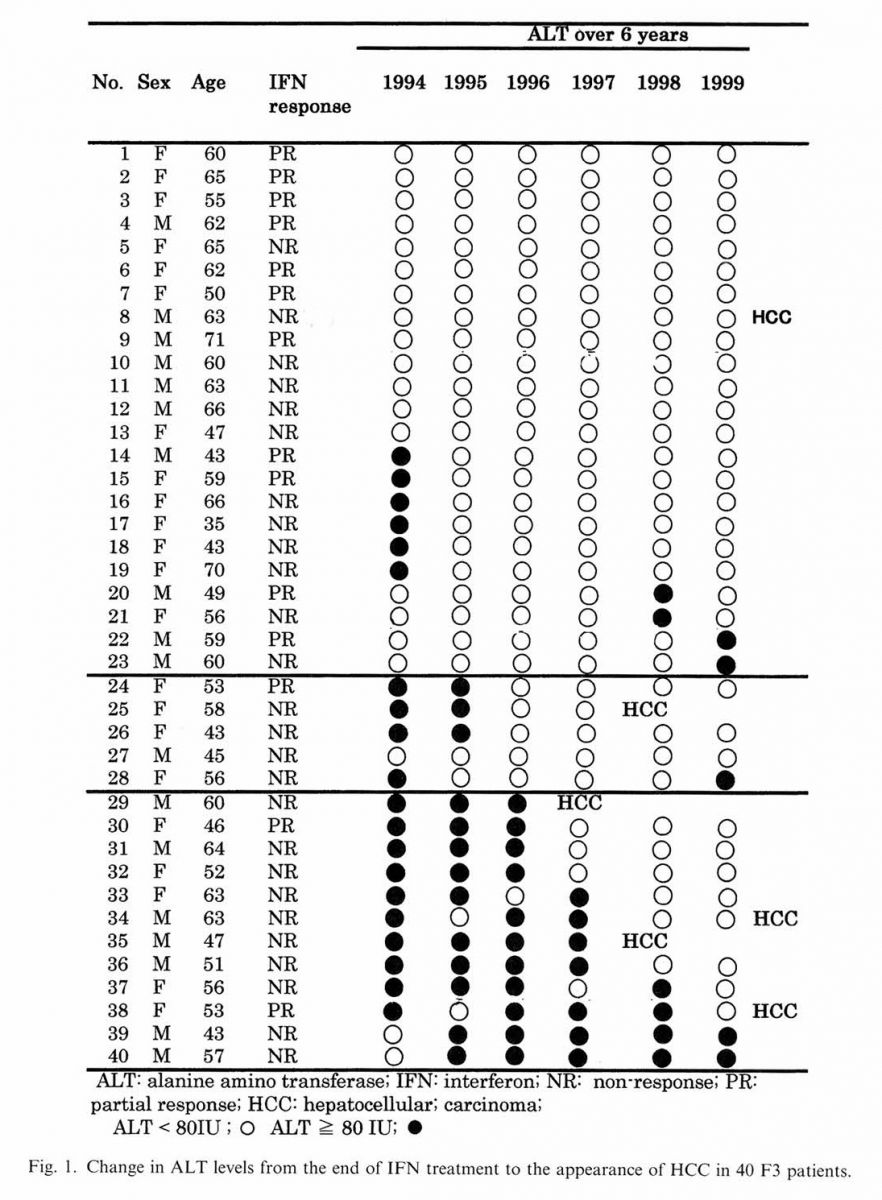

ALT 5: 80 IU for 2 years or more seemed to be at a greater risk of HCC development. In 17 out of 40 F3 patients, ALT could not be suppressed persistently below 80 IU by anti-inflammatory drugs. The reasons for this could be either host related (temporary discontinuation) or virus re¬lated. In such cases, further antiviral drug therapy with PEG-IFN and Ribavirin may be effective in bringing down high ALT activity and keeping ALT below 80 IU. CHC patients with F3 have the highest potential to progress to F4 and finally to HCC. A persistently high ALT level in CHC patients indicates continuing inflammation and suppression of ALT below 80 IU (specially during the transition from F3 to F4) may play a major role in lowering risk of HCC development. Thus we suggest continuous anti-inflammatory drug therapy to suppress ALT in non-cirrhotic CHC patients, particularly F3 group, following initial IFN therapy, to prevent or delay the appearance of HCC.
References
[1] Alter MJ. The detection, transmission and outcome of hepatitis C virus infection. Infect Agents Dis 1993;2:155 — 66.
[2] Davis GL, Balart LA, Schiff ER, Linsay K, Bodenheimer HC, Perillo RP, Carey W, et al. Treatment of chronic hepatitis C with recombinant interferon alpha: a multi-center randomized controlled trial. N Engl J Med 1989;321:1501-6.
[3] Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, et al. Association between high serum alanine aminotransferase levels and more rapid develop¬ment and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer 1999;86(4):589-95.
[4] Hagiwara H, Hayashi N, Mita E, Takehara T, Kasahara A, Fusamoto H, Kamada T. Quantitative analysis of hepatitis C virus RNA in serum during interferon alpha therapy. Gastroenterology 1993;104:877-83.
[5] Serfaty L, Giral P, Loria A, Andreani T, Legendre C, Poupon R. Factors predictive of response to interferon in patients with chronic hepatitis C. J Hepatol 1994;21:12-7.
[6] Davies GL. Prediction of response to interferon treatment of chronic hepatitis C. J Hepatol 1994;21:1-3.
[7] Pagliaro L, Craxi A, Camma C, Tine F, Di Marco V, Lo Iacono O, Almaasio P. Interferon alpha for chronic hepatitis C: an analysis of clinical predictors of response. Hepatology 1994;19:828-30.
[8] Le groupe Francais Pour L›Etude du traitement des Hepatites Chroniques NANA/C, Jouet P, Roudot-Thor-aval F, Dhumea D, Metreau J-M. Comparative efficacy of interferon alpha in cirrhotic and non-cirrhotic patients with non-A, non-B hepatitis. Gastroenterology 1994;106:686-90.
[9] Chemello L, Bonetti P, Cavelleto L, Talato F, Donadon V, Casarin P, Belussi F, et al. Randomized trial comparing three different regimens of alpha-2a-interferon in chronic hepatitis C. Hepatology 1995;22:700-6. [10] Martinot-Peignox M, Marcelhn P, Pouteau M, Castelnau C, Boyer N, Poliquin M, Degott C, et al. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alpha therapy in chronic hepatitis C. Hepatolgy 1995;22:1050-6.
[11] Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997;79(8):1494-500.
[12] Takano S, Ito Y, Yokosuka O, Ohto M, Uchimi K, Hirota K, et al. A multicenter randomized controlled dose study of ursodeoxycholic acid for chronic hepatitis C. Hepatol¬ogy 1994;20:559-64.
[13] Takikawa H, Yamanaka M, Miyake K, Kako M, Ohki H,
Narita T, et al. Ursodeoxycholic acid therapy for chronic
type C hepatitis: a multicenter, dose-finding trial. Curr
Therap Res 1994;55:16-21. ‹■, >
[14] Kiso S, Kawata S, Imai Y, Tamura S, Inui Y, Ito N, Matsuzawa Y. Efficacy of ursodeoxycholic therapy in chronic viral hepatitis C with high serum gamma-glutamic transpetidase levels. J Gastroenterol 1996;31:75-80.
[15] Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Soo RK, et al. Prospective study of chemo-prevention of hepatocellular carcinoma with sho-saiko-to (TJ9). Cancer 1995;76(5):743-9.
[16] Takahashi M, Yamada G, Miyamoto R, Doi T, Endo H, Tsuji T. Natural course of chronic hepatitis. Am J Gastroenterol 1993;88(2):240-3.
[17] Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346:1051-5.
[18] Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, et al. Risk factors for hepatocel¬lular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology 1998;27:1394-401.
[19] Kasahara A, Hayashi N, Hiramatsu N, Oshita M, Hagiwara H, Katayama K, Kato M, et al. Ability of prolonged interferon treatment to suppress relapse after cessation of patients with chronic hepatitis C: a multicenter randomized controlled trial. Hepatology 1995;21:291-7.
[20] Hiramatsu N, Hayashi N, Kasahara A, Hagiwara H, Takehara T, Haruna H, Naito M, et al. Improvement of liver fibrosis in chronic hepatitis C patients treated with natural interferon alpha. J Hepatol 1995;22:135-42.
[21] Nagayama K, Kurosaki M, Enomoto N, Miyasaka Y, Marumo F, Sato C. Characteristics of hepatitis C viral genome associated with disease progression. Hepatology 2000;31:745-50.
[22] Tarao K, Takemiya S, Tamai S, Sugumasa Y, Shinichi O, et al. Relationship between the recurrence of hepatocel¬lular carcinoma (HCC) and serum alaninie aminotransfer¬ase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer 1997;79(4):688-94.
[23] Fong T, Shindo M, Feinstone SM, Hoofnagle JH, Di Biscglie AM. Detection of replicative intermediates of hepatitis C viral RNA in liver and serum of patients with chronic hepatitis C. J Clin Invest 1991;88:1058-60.
[24] Mangia A, Vallari DS, Di Bisceglie AM. Use of con¬firmatory assays for diagnosis of heptitis C viral infection in patients with hepatocellular carcinoma. J Med Virol 1994;43:125-8.
[25] Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 1998;4(9):1065-7.
[26] Nishikawa M, Nishiguchi S, Shiomi S, Tamori A, Koh N, Takeda T, et al. Somatic mutations of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Res 2001;61:1843-5.
[27] Tarao K, Rino Y, Takemiya S, Tamai S, Ohkawa S, Sugimasa Y, et al. Close association between high serum ALT and more rapid recurrence of hepatocellular carci¬noma in hepatectomized patients with HCV-associated liver cirrhosis and hepatocellular carcinoma. Intervirology 2000;43(l):20-6.
Нийтлэлийн нээгдсэн тоо: 951




